Describe in Words the Meaning of Ka the Dissociation Constant
What does dissociation constant mean. The action of disconnecting or separating or the state of being disconnected.
Ka is the acid dissociation constant.

. There are tables of acid dissociation constants for easy reference. They have an inverse relationship. PKa is the -log of Ka having a smaller comparable values for analysis.
The action of disconnecting or separating or the state of being disconnected. The first second third etc. Ionization constant symbol K Learn More About dissociation constant.
A rejection or denial of a belief or preconception. Show activity on this post. A constant that depends upon the equilibrium between the dissociated and undissociated forms of a chemical combination especially.
K a is commonly expressed in units of molL. The dissociation constant of water is denoted K w. Here are all the possible meanings and translations of the word dissociation constant.
Dissociation constant noun Chemistry A quantity expressing the extent to which a particular substance in solution is dissociated into ions equal to the product of the concentrations of the respective ions divided by the concentration of the undissociated molecule. A breach of a harmonious relationship. The k denotes the word constant.
This equilibrium constant is a quantitative measure of the strength of an acid in a solution. Although the rate constants ka and kd are specific for a specific ligand-analyte pair they are independent of the concentration of both ligand and analyte. Acid dissociation constants refer to the equilibrium constant for loss of the first second third and so on proton.
To learn more about Calculation of pka List of pKa values Relationship between pKa and pH and FAQs of pKa Visit BYJUS. Units for k on are units of D multiplied by the units of R over time ie. Dissociation constant - the equilibrium constant for a reversible dissociation equilibrium constant - chemistry the ratio of concentrations when equilibrium is reached in a reversible reaction when the rate of the forward reaction equals the rate of the reverse reaction.
What does dissociation constant mean. The acid and base dissociation constants are usually expressed in terms of moles per liter molL. For example H 2 S O 4 can lose one proton to make HSO4 which can then lose another proton to generate SO42.
This variation must be taken into account when making precise measurements of. Typically we determine the dissociation constant by seeing how much of. It is the concentration in Molar of analyte where 50 of the ligand is occupied by the analyte in a 1 to 1 interaction.
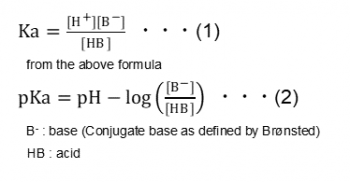
PKa - The pKa value is the negative base -10 logarithm of the acid dissociation constant Ka of a solution. 3pts for each correct expression 1 pH ii pKa c The acid dissociation constant K for acetic acid to form acetate is roughly 10 M. Meaning of dissociation constant.
The acid dissociation constant is the equilibrium constant of the dissociation reaction of an acid and is denoted by K a. The equilibrium constant for a reversible dissociation. 2 Background The acid dissociation constant K a of a drug is a property of fundamental importance in medicinal chemistry because it determines much about how the drug is absorbed into the body where that drug will then distribute within body and finally what metabolic mechanisms will result in the excretion of the drug.
K D is the dissociation constant and is the concentration of ligand which half the ligand binding sites on the protein are occupied in the system equilibrium. How to calculate your KD ratio. To prepare a 10 mmolL pH 5 acetic acidsodium acetate buffer how many This problem has been solved.
Similarly Kb is the base dissociation constant while pKb is the -log of the constant. The equilibrium constant involved in the dissociation of a compound into two or more compounds or ions. DISSOCIATION CONSTANT noun The noun DISSOCIATION CONSTANT has 1 sense.
PKa is simply the -log of this constant. The value of K w varies with temperature as shown in the table below. The dissociation constant is the ratio of dissociated ions products to original acid reactants.
The extent of dissociation is indicated by the value of the acid dissociation constant or K a which is calculated as 𝐾𝐾𝑎𝑎 𝐻𝐻3𝑂𝑂𝐶𝐶𝐻𝐻3𝐶𝐶𝑂𝑂 𝐶𝐶𝐻𝐻3𝐶𝐶𝑂𝑂𝑂𝑂𝐻𝐻 3 The square brackets indicate the molar concentrations of the products and reactants at equilibrium. Ka is acid dissociation constant and represents the strength of the acid. The dissociation constant or also known as kd is reverse of the freezing point depression constant also known as kf.
How do you calculate KD. It is calculated by dividing the k off value by the k on value. The concentration of water is omitted by convention which means that the value of K w differs from the value of K eq that would be computed using that concentration.
Ka AH HA K a A H H A. Rate constants k on and k off and the dissociation constant K d k on is the rate constant of association. The equilibrium constant for a reversible dissociation.
It is abbreviated as Ka. This is typically covered in a general chemistry course. The dissociation constant is usually written as a quotient of the equilibrium concentrations in molL.
Ka1 and Ka2 would be the equilibrium constants for. The equilibrium dissociation constant has a clear meaning. An acid dissociation constant K a is a quantitative measure of the strength of an acid in solution.
The quantitative behavior of acids and bases in solution can be understood only if their pKa values are known. Princetons WordNet 000 0 votes Rate this definition. Larger the Ka smaller the pKa and stronger the acid.
The reciprocal of the association constant. It is usually expressed in moles per second or picomoles per hour depending on how lazy your reaction rate and how scarce the reagents. KDA kills assists deaths for your kill-deathsassists ratio.
K off is the rate constant of dissociation.

What Is An Equilibrium Constant Chemistry Lessons Chemistry Classroom Study Chemistry
How Should The Acid Dissociation Constant Pka Be Measured Automatic Potentiometric Titrators Faq Kyoto Electronics Manufacturing Co Ltd Kem

No comments for "Describe in Words the Meaning of Ka the Dissociation Constant"
Post a Comment